100763
论文已发表
提 交 论 文
注册即可获取Ebpay生命的最新动态
注 册
IF 收录期刊
- 3.3 Breast Cancer (Dove Med Press)
- 3.4 Clin Epidemiol
- 2.5 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.5 Clin Interv Aging
- 4.7 Drug Des Dev Ther
- 2.7 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.5 Int J Women's Health
- 2.5 Neuropsych Dis Treat
- 2.7 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.3 Ther Clin Risk Manag
- 2.5 J Pain Res
- 2.8 Diabet Metab Synd Ob
- 2.8 Psychol Res Behav Ma
- 3.0 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.7 Risk Manag Healthc Policy
- 4.2 J Inflamm Res
- 2.1 Int J Gen Med
- 4.2 J Hepatocell Carcinoma
- 3.7 J Asthma Allergy
- 1.9 Clin Cosmet Investig Dermatol
- 2.7 J Multidiscip Healthc

已发表论文
壳聚糖修饰的纳米硒作为蛋白载体提高对 2 型糖尿病的肽治疗 BAY55-9837 的体内半衰期
Authors Rao L, Ma Y, Zhuang MJ, Luo TJ, Wang YY, Hong A
Published Date October 2014 Volume 2014:9(1) Pages 4819—4828
DOI http://dx.doi.org/10.2147/IJN.S67871
Received 16 May 2014, Accepted 24 June 2014, Published 17 October 2014
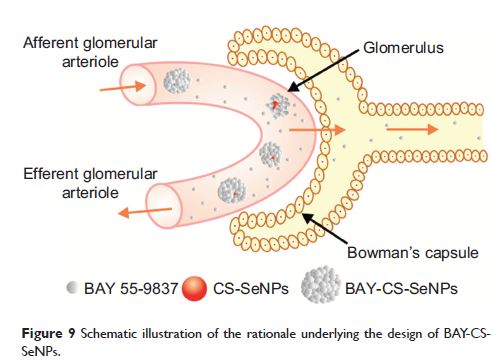
Purpose: As a potential protein therapeutic for type 2 diabetes mellitus (T2DM), BAY 55-9837 is limited by poor stability and a very short half-life in vivo. The purpose of this study was to construct a novel nanostructured biomaterial by conjugating BAY 55-9837 to chitosan-decorated selenium nanoparticles (CS-SeNPs) to prolong the in vivo half-life of BAY 55-9837 by reducing its renal clearance rate.
Materials and methods: BAY 55-9837-loaded CS-SeNPs (BAY-CS-SeNPs) were prepared, and their surface morphology, particle size, zeta potential, and structure were characterized. The stability, protein-loading rate, and in vitro release of BAY 55-9837 from CS-SeNPs were also quantified. Additionally, a sensitive high-performance liquid chromatography (HPLC) assay was developed for the quantification of BAY 55-9837 in mouse plasma. Thereafter, mice were injected via the tail vein with either BAY 55-9837 or BAY-CS-SeNPs, and the plasma concentration of BAY 55-9837 was determined via our validated HPLC method at different time intervals postinjection. Relevant in vivo pharmacokinetic parameters (half-life, area under the curve from time 0 to last sampling point, observed clearance) were then calculated and analyzed.
Results: BAY-CS-SeNPs were successfully synthesized, with diameters of approximately 200 nm. BAY-CS-SeNPs displayed good stability with a high protein-loading rate, and the release process of BAY 55-9837 from the CS-SeNPs lasted for over 70 hours, with the cumulative release reaching 78.9%. Moreover, the conjugation of CS-SeNPs to BAY 55-9837 significantly reduced its renal clearance to a rate of 1.56 mL/h and extended its half-life to 20.81 hours.
Conclusion: In summary, our work provides a simple method for reducing the renal clearance rate and extending the half-life of BAY 55-9837 in vivo by utilizing CS-SeNPs as nanocarriers.
Keywords: BAY 55-9837, selenium nanoparticles, clearance, half-life, type 2 diabetes
Materials and methods: BAY 55-9837-loaded CS-SeNPs (BAY-CS-SeNPs) were prepared, and their surface morphology, particle size, zeta potential, and structure were characterized. The stability, protein-loading rate, and in vitro release of BAY 55-9837 from CS-SeNPs were also quantified. Additionally, a sensitive high-performance liquid chromatography (HPLC) assay was developed for the quantification of BAY 55-9837 in mouse plasma. Thereafter, mice were injected via the tail vein with either BAY 55-9837 or BAY-CS-SeNPs, and the plasma concentration of BAY 55-9837 was determined via our validated HPLC method at different time intervals postinjection. Relevant in vivo pharmacokinetic parameters (half-life, area under the curve from time 0 to last sampling point, observed clearance) were then calculated and analyzed.
Results: BAY-CS-SeNPs were successfully synthesized, with diameters of approximately 200 nm. BAY-CS-SeNPs displayed good stability with a high protein-loading rate, and the release process of BAY 55-9837 from the CS-SeNPs lasted for over 70 hours, with the cumulative release reaching 78.9%. Moreover, the conjugation of CS-SeNPs to BAY 55-9837 significantly reduced its renal clearance to a rate of 1.56 mL/h and extended its half-life to 20.81 hours.
Conclusion: In summary, our work provides a simple method for reducing the renal clearance rate and extending the half-life of BAY 55-9837 in vivo by utilizing CS-SeNPs as nanocarriers.
Keywords: BAY 55-9837, selenium nanoparticles, clearance, half-life, type 2 diabetes
Download Article[PDF]